REVIEW QUESTIONS
Q76.
At
approximately
what
temperature
(Fahrenheit) would you expect rime ice to form
on a ship, assuming blowing spray is present?
Q77. What elements are included in an ice accretion
observation?
ICE IN THE SEA
LEARNING OBJECTIVES: Explain the
importance of sea ice to naval operations.
Describe the various sea ice classifications, sea
ice sizes, and topography of sea ice sheets.
Discuss movement of sea ice and ice of land
origin. Explain the judgments to be made when
observing ice in the sea.
Roughly three percent of the world’s water areas are
covered in ice. Although small in area, the ice-covered
areas of the sea and oceans are important to naval
operations because of their proximity to possible hostile
forces. Many submarines routinely operate beneath the
ice, and surface ships occasionally operate in ice-
covered seas or areas frequented by icebergs. The
Naval Ice Center in Suitland, Maryland, keeps the Fleet
advised of the development, movement, and
equatorward limit of the ice edge, as well as of the
location and movement of icebergs. Although they
make extensive use of satellite imagery to detect and
track ice, the ice observations from ships operating near
the ice provide valuable input to this critical tracking
and forecasting effort. Observations of ice seen floating
in the sea are completed as part of each surface weather
observation.
There are two main types of ice found floating in the
sea: sea ice and ice of land origin.
SEA ICE
Sea ice is ice that forms in the sea. It is, for the most
part, frozen seawater. Sea ice accounts for
approximately 95% of the ice coverage in the oceans.
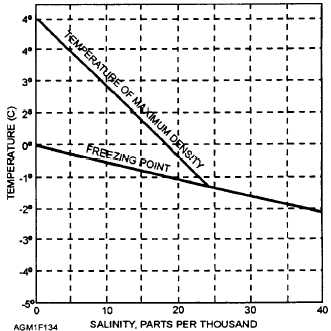
For seawater to freeze, the temperatures must be
colder for a longer period of time. This is due to the
salinity of the water and because of the density changes
in the water caused by the salinity. We know that pure
water freezes at 0°C (32°F) but the freezing point of
seawater varies, depending on the salinity (fig. 1-34).
Seawater averages 35‰ or 35 parts per thousand by
weight salinity. With this salinity, water begins to
freeze at -1.9°C (28°F). Freshwater reaches maximum
density at 4°C (39°F). In effect, as freshwater ponds and
lakes cool, and the surface waters reach 4°C the water
sinks and warmer subsurface water rises to replace it.
This slows the process of cooling the surface of the pond
below 4°C until the entire body of water is cooled to
4°C. After this point, surface waters cooled to less than
4°C are slightly less dense than the water below the
surface, and cooling to the freezing point is rapid.
Seawater on the other hand, reaches maximum density
at the freezing point. When surface seawater is cooled to
the freezing point, but before ice can form, the water
sinks and is replaced from below by slightly warmer
water. The overturn process continues for a long period
of time, even in continued subfreezing air temperatures,
until a large column of water can be cooled. Overall, the
lower freezing point and greater overturn required
makes the freezing process of seawater very slow. The
freezing of seawater is further retarded by the mixing
action of winds (waves), currents, and tides. Once ice
forms, it floats. Ice, even saltwater ice, expands as it
freezes, so it is less dense than water at the same
temperature.
The formation of sea ice usually begins with the
onset of autumn, and the first ice usually appears in the
mouths of rivers that empty into shallow seas, such as
that off northem Siberia. During the increasingly longer
and colder nights of autumn, ice forms along the
shorelines (fast ice) and becomes a semipermanent
Figure 1-34.—Freezing point and temperature of maximum
density versus water salinity.
1-50