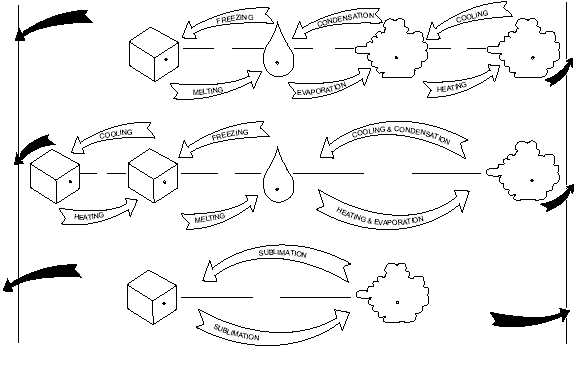
Liquid to Gas and Vice Versa
Water undergoes the process of evaporation when
changing from the liquid to a gaseous state. According
to the molecular theory of matter, all matter consists of
molecules in motion. The molecules in a bottled liquid
are restricted in their motion by the walls of the
container. However, on a free surface exposed to the
atmosphere, the motion of the molecules in the liquid is
restricted by the weight of the atmosphere or, more
precisely, by the atmospheric pressure. If the speed of
the liquid molecules is sufficiently high, they escape
from the surface of the liquid into the atmosphere. As
the temperature of the liquid is increased, the speed of
the molecules is increased, and the rate at which the
molecules escape from the surface also increases.
Evaporation takes place only from the free or exposed
surface of a substance.
During the process of evaporation, heat is released.
This heat is absorbed by the water that has vaporized.
The amount absorbed is approximately 539 calories per
gram of water at a temperature of 100°C. On the other
hand, the amount is 597.3 calories, if the evaporation
takes place at a water temperature of 0°C. This energy
is required to keep the molecules in the vapor state and
is called the latent heat of vaporization. Since the water
needs to absorb heat in order to vaporize, heat must be
supplied or else evaporation cannot take place. The air
provides this heat. For this reason, evaporation is said to
be a cooling process, because by supplying the heat for
vaporization, the temperature of the surrounding air is
lowered.
Condensation is the opposite of evaporation
because water vapor undergoes a change in state from
gas back to liquid. However, a condition of saturation
must exist before condensation can occur. That is, the
air must contain all the water vapor it can hold (100
percent relative humidity) before any of it can condense
from the atmosphere. In the process of condensation,
the heat that was absorbed in evaporation by the water
vapor is released from the water vapor into the air and is
called the latent heat of condensation. As you might
expect, condensation warms the surrounding air.
Solid to Gas and Vice Versa
Sublimation is the change of state from a solid
directly to a vapor or vice versa at the same
temperature. In physics and chemistry, sublimation is
regarded as the change of state from solid to vapor only,
2-7
1 gm
ICE
0 C
1 gm
ICE
0 C
1 gm
ICE
-10 C
1 gm
ICE
0 C
1 gm
INVISIBLE
WATER VAPOR
100 C
1 gm
INVISIBLE
WATER VAPOR
100 C
1 gm
WATER
0 C
1 gm
WATER
0 C
1 gm
INVISIBLE
WATER VAPOR
0 C
TOTAL CALORIES
ADDED TO
THE AIR
727
723
677
TOTAL CALORIES
TAKEN FROM
THE AIR
727
723
677
80
597
50
4
80
100+539
677
NOTE:
1. EVAPORATION COOLS AIR.
2. CONDENSATION HEATS.
3. CALORIES SHOWN TO NEAREST
WHOLE FIGURES.
1 gm
INVISIBLE
WATER VAPOR
0 C
AG5f0205
Figure 2-5.—Thermal history of 1 gram of ice during changes of state.