For instance, water vapor in the atmosphere is
independent of the presence of other gases. The vapor
pressure is independent of the pressure of the dry gases
in the atmosphere and vice versa. However, the total
atmospheric pressure is found by adding all the
pressures—those of the dry air and the water vapor.
TERMS
The actual amount of water vapor contained in the
air is usually less than the saturation amount. The
amount of water vapor in the air is expressed in several
different methods. Some of these principal methods are
described in the following portion of this section.
Relative Humidity
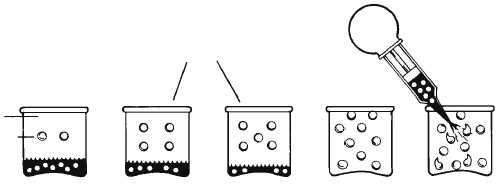
Although the major portion of the atmosphere is
not saturated, for weather analysis it is desirable to be
able to say how near it is to being saturated. This
relationship is expressed as relative humidity. The
relative humidity of a volume of air is the ratio (in
percent) between the water vapor actually present and
the water vapor necessary for saturation at a given
temperature. When the air contains all of the water
vapor possible for it to hold at its temperature, the
relative humidity is 100 percent (See fig. 1-12).
A
relative humidity of 50 percent indicates that the air
contains half of the water vapor that it is capable of
holding at its temperature.
Relative humidity is also defined as the ratio
(expressed in percent) of the observed vapor pressure to
that required for saturation at the same temperature and
pressure.
Relative humidity shows the degree of saturation,
but it gives no clue to the actual amount of water vapor
in the air. Thus, other expressions of humidity are
useful.
Absolute Humidity
The mass of water vapor present per unit volume of
space, usually expressed in grams per cubic meter, is
known as absolute humidity. It may be thought of as the
density of the water vapor.
Specific Humidity
Humidity may be expressed as the mass of water
vapor contained in a unit mass of air (dry air plus the
water vapor). It can also be expressed as the ratio of the
density of the water vapor to the density of the air
(mixture of dry air and water vapor). This is called the
specific humidity and is expressed in grams per gram or
in grams per kilogram. This value depends upon the
measurement of mass, and mass does not change with
temperature and pressure. The specific humidity of a
parcel of air remains constant unless water vapor is
added to or taken from the parcel. For this reason, air
that is unsaturated may move from place to place or
from level to level, and its specific humidity remains
the same as long as no water vapor is added or removed.
However, if the air is saturated and cooled, some of the
water vapor must condense; consequently, the specific
humidity (which reflects only the water vapor)
decreases. If saturated air is heated; its specific
humidity remains unchanged unless water vapor is
added to it. In this case the specific humidity increases.
The maximum specific humidity that a parcel can have
occurs at saturation and depends upon both the
temperature and the pressure. Since warm air can hold
more water vapor than cold air at constant pressure, the
saturation specific humidity at high temperatures is
1-20
THE DIFFERENCE BETWEEN
ACTUAL TEMP. AND DEW POINT
TEMP. IS AN INDICATION OF
HOW CLOSE THE AIR IS TO
SATURATION.
IF COOLED TO DEW POINT
TEMP. OR ADDITIONAL
WATER VAPOR IS ADDED
TO SATURATED AIR,
CONDENSATION OCCURS.
DRY AIR
WATER VAPOR
AIR TEMP
DEW POINT
RELATIVE HUMIDITY
40
60
80
80
80
80
80
40
100
100
100
100
60
60
50
O
O
O
O
O
O
O
O
O
O
O
O
%
%
%
%
%
F
F
AGf0112
Figure 1-12.—Relative humidity and dew point.