The quantity of water vapor needed to produce
saturation does not depend on the pressure of other
atmospheric gases. At a given temperature, the same
amount of water vapor saturates a given volume of air.
This is true whether it be on the ground at a pressure of
1000 mb or at an altitude of 17,000 ft (5,100 meters)
with only 500 mb pressure, if the temperature is the
same. Since density decreases with altitude, a given
volume of air contains less mass (grams) at 5,100
meters than at the surface. In a saturated volume, there
would be more water vapor per gram of air at this
altitude than at the surface.
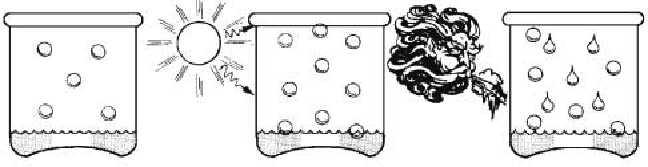
Temperature
Although the quantity of water vapor in a saturated
volume of atmosphere is independent of the air
pressure, it does depend on the temperature. The higher
the temperature, the greater the tendency for liquid
water to turn into vapor. At a higher temperature,
therefore, more vapor must be injected into a given
volume before the saturated state is reached and dew or
fog forms. On the other hand, cooling a saturated
volume of air forces some of the vapor to condense and
the quantity of vapor in the volume to diminish.
Condensation
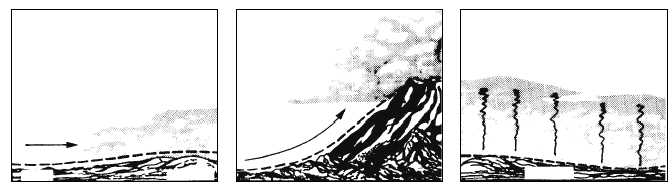
Condensation occurs if moisture is added to the air
after it is saturated, or if cooling of the air reduces the
temperature below the saturation point. As shown in
figure 1-11, the most frequent cause of condensation is
cooling of the air from the following results: (a) air
moves over a colder surface, (b) air is lifted (cooled by
expansion), or (c) air near the ground is cooled at night
as a result of radiation cooling.
Pressure (Dalton’s Law)
The English physicist, John Dalton, formulated the
laws relative to the pressure of a mixture of gases. One
of the laws states that the partial pressures of two or
more mixed gases (or vapors) are the same as if each
filled the space alone. The other law states that the total
pressure is the sum of all the partial pressures of gases
and vapors present in an enclosure.
1-19
SATURATED
CONDENSING
HEAT
COOL
60 F
o
80 F
o
60 F
o
AGf0110
Figure 1-10.—Saturation of air depends on its temperature.
WARM
COLDER
COLDER
AIR MOVES IN OVER
COLDER SURFACE.
COOLED BY
EXPANSION.
RADIATION COOLING
LIFTING
A
B
C
AGf0111
Figure 1-11.—Causes of condensation.